Fame
Fecal Autologous Microbiota Expansion
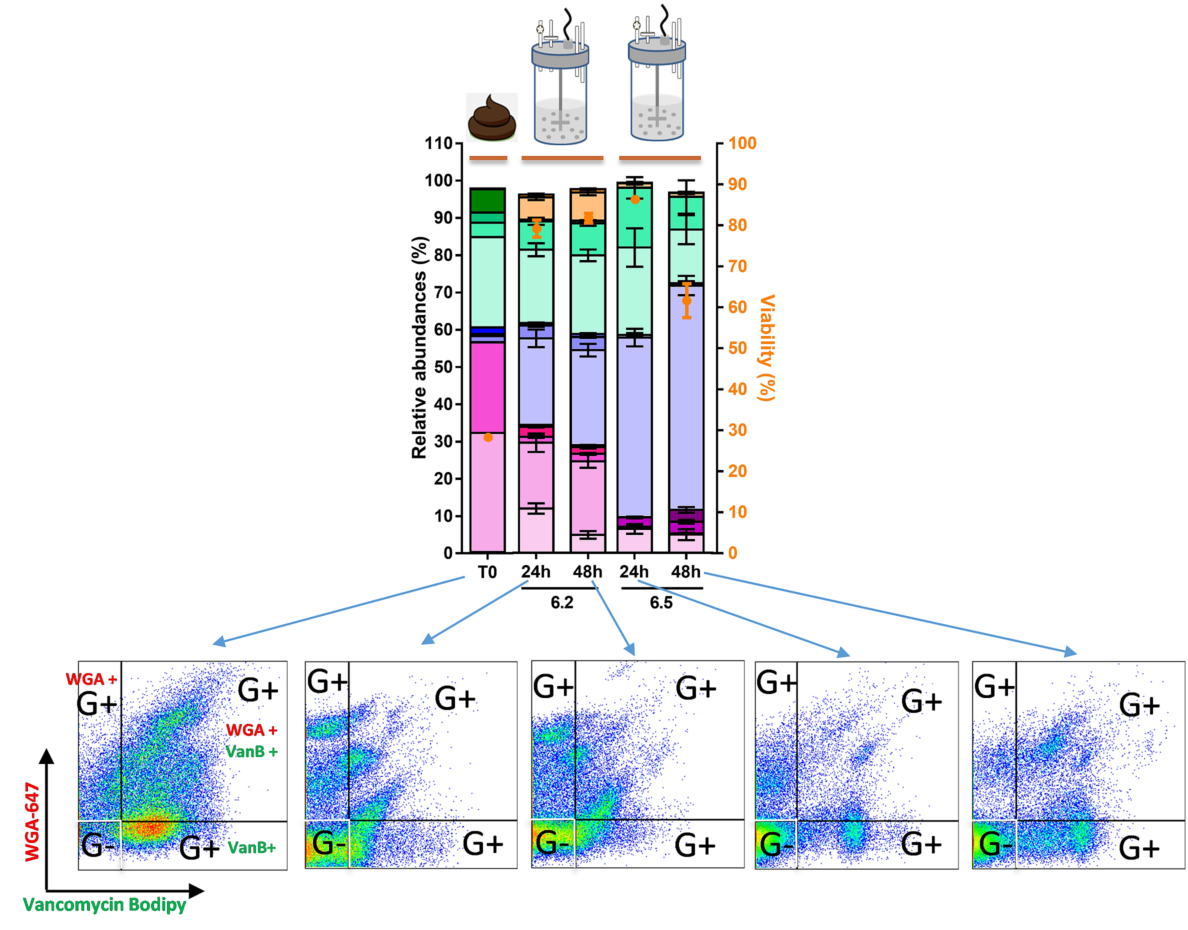
Case Study
Fecal Autologous Microbiota Expansion
The microbiota company MaaT Pharma develops secured and standardized microbiotherapeutic solutions (fecal transfer) for enteropathic dysbiosis
A joint PhD project was developed between BIOASTER and MaaT Pharma.
The main objectives of the project were: