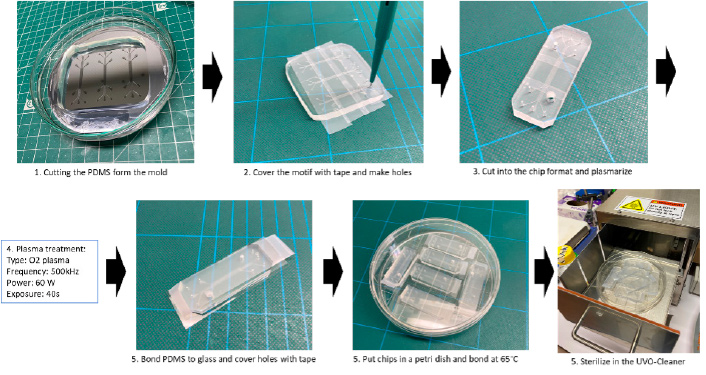
Evidence / Results
We developed a PDMS-glass microfluidic device that spatially organizes a co- culture consisting of primary human skeletal and endothelial cells mimicking the interface between blood flow and a microtissue. An injectable biodegradable,
a natural hydrogel, is introduced in-between both circulation and microtissue compartments to maintain tissue integrity. To simulate in vivo-like conditions, shear stress can be applied to circulating PBMCs in a physiological range. An ultimate objective is to achieve shear stress up to 30 dyn/cm2..
Advantages
In addition to the advantages of any organ on chip, the technology benefit of the following features:
- Being a dynamic system applying shear stress to cells
- No pre-requisite according to the adjuvant formulation chemical class
On a more technical point of view the advantage of the technology regarding inflammation in tissue is to
- Confine biodegradable hydrogel in microchannel.
- Demonstrate the molecular transport of through the hydrogel.
- Demonstrate the possibility of Human cell co-culture
Potential Applications
All application where there is a need for better in vitro models and where cells are in motion and in controlled O2 tension may be targeted.
More precisely, application will focus on any research that requires to measure and monitor early time point and innate response.
On a first approach, the technology will be designed to better characterize and classify the molecular determinants of inflammation and its resolution associated with different types of adjuvants. This will pave the way to improve vaccine design and access to personalized vaccinology.
Outlook
Collaboration with owners of adjuvants is thought to enter into the second validation phase using commercial adjuvants to compare RNA Seq data obtained with the technology to already published technologies. Some adjuvants may not be compatible with the Bood-on-Chip device. There may be a design phase depending on demand.
Discussion for a demonstration on clinical sample are currently occurring, and any industrial partner interested to the development of the Blood-On-chip technology main join this demonstration phase.
A second version of the technology will deepen the biological aspect and will integrate lymphoid like organs to take immune response a step further.