Advantage
- Simple technology requiring no specialist knowledge once databases completed
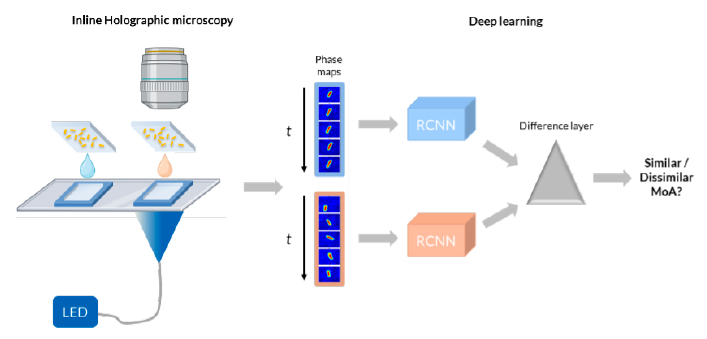
- Label-free technology based on digital inline holographic microscopy • Live bacteria, so time-course of measurements possible
- Time-frame for measurement: 0 to >24 h, typically 2 h
- Medium-throughput, semi-automated platform
- Simpler, faster, less expensive than classical methods
- Provide the potential MoA during preclinical development with good accuracy • Detect the novelty of the MoA of new antimicrobials in development
Potential Applications
HoloMoA has the potential to facilitate & accelerate new antimicrobial preclinical drug development from discovery to lead up optimization by:
- Providing a decision tool to allow selection of a series of molecules to go into lead optimization
- Potentially characterize the novelty of the mechanism of action of candidate antimicrobials
- Potential to expand the database to many other target pathogens, including fungi and other eukaryotic pathogens
- Potential to expand studies to phenotypic changes to mammalian cells to facilitate toxicity analysis and antiviral screening
- Potential to combine technology with other phenotypic screening technologies in development at Bioaster – potentially on the same cells, not just the same sample
Outlook
BIOASTER is seeking partners aiming to work together on the future development of the HoloMoA project:
- Test more compounds with different MoA, compound combinations and compounds with novel MoA, to expand the database, improve the algorithm/s and enhance statistical significance of the data
- Expand the scope to other target pathogens, including Gram positive bacteria, ESKAPE pathogens, parasites and fungi
- Extend the technology to assess phenotypic changes to mammalian cells, allowing the potential for toxicity screening, antiviral studies and studies involving intracellular pathogens
- Create stand-alone units with potential diagnostic or laboratory equipment manufacturers
- Combine the technology with other phenotypic screening technologies develop by partners or existing within BIOASTER